<Research Projects>
1 Creation of Artificial (Metallo)proteins Based on Structural/Functional Characteristics of Parent Proteins
Metalloproteins regulate the reactivities of metal ions included in protein matrices by coordination of amino acid residues and/or cofactors to exhibit the intrinsic characteristics. Chemical modification and genetic mutation of naturally occurring proteins are versatile strategies to develop artificial proteins with unique functions. Furthermore, targeting functions which are not seen in naturally occurring systems are also a challenging issue.
The basic structural unit of proteins is L-amino acid. Highly-ordered structures (alpha-helix and beta-sheet structures) provided by L-amino acid polymers are essential for building-up specific and unique reaction centers in protein matrices. For example, the highly-ordered protein structures contribute to optimization of positioning of functional groups in reaction center of enzymes to efficiently catalyze chemical reactions and to exhibit substrate-selectivity. Our research group investigates the strategies of protein engineering in which the structural/functional features of original proteins are fully employed.
Creation of olefin metathesis-catalyzing artificial metalloenzyme
Most of matalloprotein designs have targeted metal complexes with N-, O- or S-coordination (known as "inorganic complexes) provided by amino acid residue side chains and/or organic cofactors such as heme. C-Coordinated metal complexes (organometallic complexes) are also possible tools for constructions of artificial metalloproteins. Furthermore, one of the important points in protein engineering is "targeting functions".
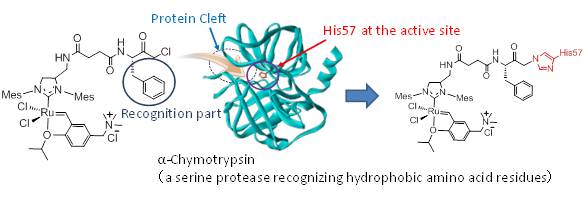
We developed a protease inhibitor equipped with a Hoveyda-Grubbs complex moiety and regioselectively introduced it into the structural cleft of alpha-chymotrypsin, a serine protease. The prepared protein functions as a biocatalyst for olefin metathesis.
【in detail】Chem. Commun. 2012 ,48, 1662-1664; Catalysts 2021, 11, 359
Creation of "Protein-based Molecular Machines"
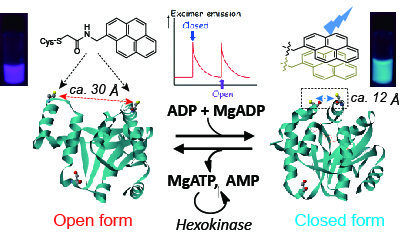
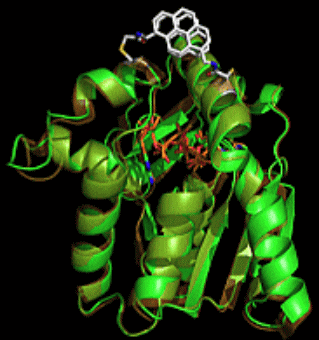
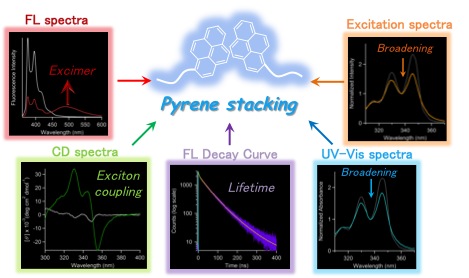
Some enzymes show large conforimational changes on substrate binding/product release to exhnit their intrinsic functions. Adenylate kinase, a phosphoryl transfer-mediating enzyme, undergoes large coformtional changes on the binding ADP and the release of ATP and AMP. The protein dynamics will be applied for construntion of unique function swtiching system triggered by enzymatic reactions after conjugation of synthetic molecules on the protein surface. As an example, we conjugated pyrene compounds onto the surface of adenylate kinase and successfully constructed a pyrene photo-property switching sysyem in response to the catalytic cycle of adenylate kinase, which is a "protein-based molecular machine" with function swithcing triggered by small molecule binding. Furthermore, we experimentally investigated a correlation between pyrene stacking mode, pyrene probe structures and emission spectral properties on the protein surface using X-ray crystallography and emission spectral measurements.
【in detail】 Bioconjugate Chem. 2013, 24, 1218-1225; Bioconjugate Chem. 2015, 26, 537.
2 Development of Olefin-metathesis Catalysts through Ligand Modficiation
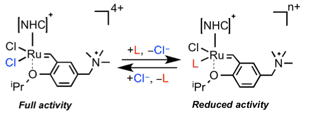
Inspired by the work on the olefin metathesis artificial enzyme shown above, we have also attempted to develop olefin metathesis as a biochemical research protocol. Normally, Hoveyda-Grubbs-type complexes are readily deactivated in water. In this context, we have demonstrated the utility of additive chloride salt in aqeuos olefin metathesis reactions.
【in detail】Organometallics 2013, 32, 5313.
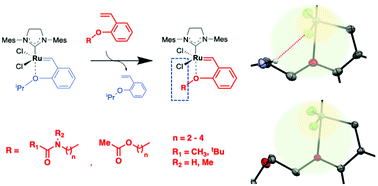
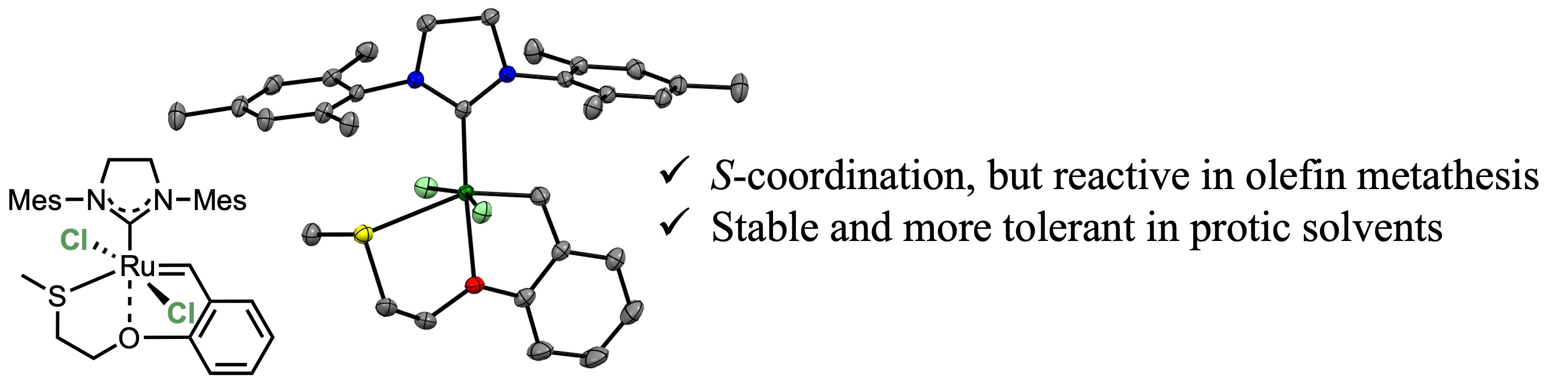
Moreover, we have attempted to regulate the reactivities of Hoveyga-Grubbs-type complexes by structural modification of the benzylidene ligand, where we have demonstrated the reactivitiy regulation of the complexes through second-coordination sphere effects and through chalcogen atom effects.
【in detail】Daltron Trans. 2020, 49, 11618; Chem. Lett. 2020, 49, 1490; Daltron Trans. 2023, 52, 9499.
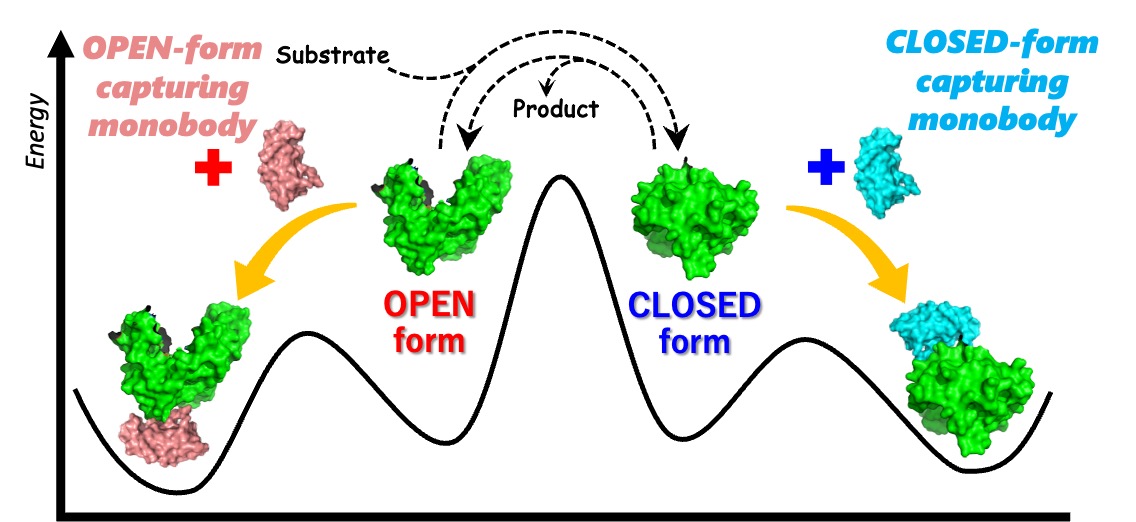
3. Function Regulation of Kinases with Monobodies (synthetic binding proteins)
Related with the project of protein-based molecular machines, we have developed monobodies (small-size synthetic binding proteins) that recognize the OPEN/CLOSED forms of adenylate kinase (This is a collaborative work with a research group of Prof. Shun-ichi Tanaka at Kyoto Prefetural University). We have investigated the action mechanism of the monobdies for regulating the function of adenylate kinase.
【In details】Protein Sci. 2023, 2023, 32, e4813.
4. Action Mechanism of Bio-active Molecules for Control of Biochemical Reactions
Some small bio-active molecules are attractive for control of biochemical reactions (e.g. apoptosis, amyloid formation etc,). Clarification of the action mechanism of these compounds is essential for medical apllications. We have attempted to clarify the interactions between proteins and bio-active molecules by biochemical strategy to obtain molecular information for development of synthetic compounds which can be applied for medical treatments. Especially, we are interested in the action mechanism of apoptosis-inducing small organic compounds. Recently, we found that PAC-1, an apoptosis-inducing reagent in cancer cells, is able to enhance the catalytic activity of mature caspase-3 as well as recovery of caspase maturation from the corresponding zymogen.
【in detail】 Bull. Chem. Soc. Jpn. 2015, 88, 1221.(BCSJ award article
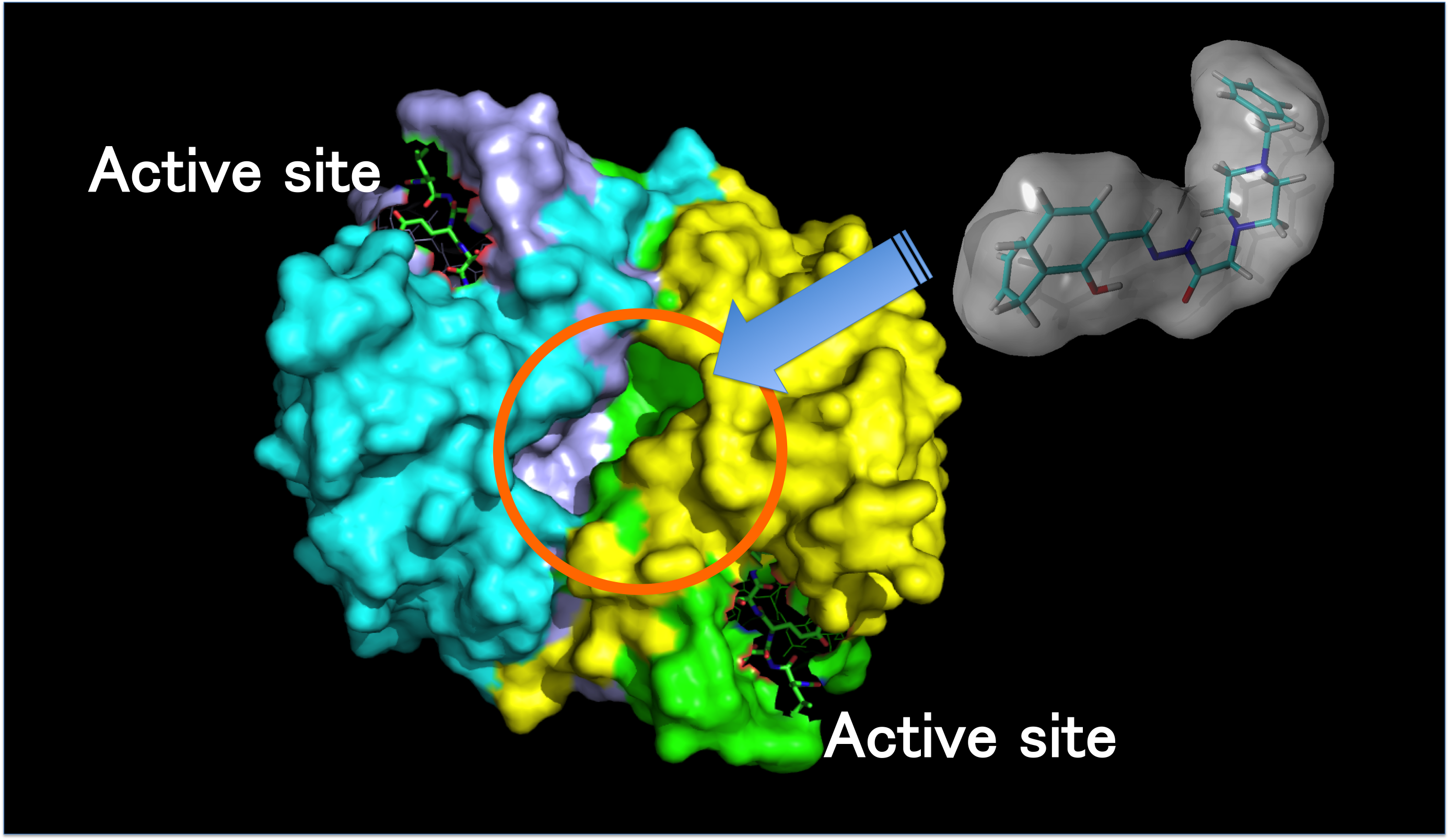

5. Why is a huge protein structure required to exhibit the intrinsic functions of metal-containing proteins?: Clarification of regulation mechanism for metal ions bound to protein core
Functions of metal-containing proteins originate from the propertites of metal ions bound to the protein core. Metal ions at the active centers are relatively small compared to protein core. Herein, a basic and important question will be raised: Why do metal-containing proteins have a huge structure to control the properties of small metal ions? We investigated the problem using thiol-subtiilisin, a model protein, and recently proposed that global structural flexibility provided by proteins as amino acid polymers should be considered to figure out the regulation mechanism of metal ions bound to the protein core.
【in detail】 Chem. Eur. J. 2018, 24, 2767.

6. Organometallic Reactions Mediated by Metalloporphyrin-related Complexes
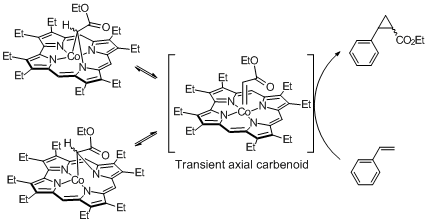
Porphyrin is a pi-conjugated macrocycle in which four pyrrole rings are linked by methylene bridges ("meso"-position). The characteristic pi-conjugation property is important to efficiently control the reactivities of the centered metal. Porphyrin-related complexes with different arrangements of four pyrrole units will be expected to obtain chemical knowledge which are difficult to clarify by simple modification of functional groups in the periphery of the porphyrin macrocycle.
Cobalt (III) corrole (lacking one meso position of porphyrin framework) reacts with ethyl diazoacetate to transiently generate the corresponding carbene intermediate. The carbenoid easily changed into N-bridged species. We experimentally found that the two N-bridged species are in equilibrium in a solution.
【In detail】Organometallics. 2011, 30, 1869-1873
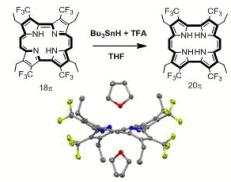
7. Chemistry of Porphyrinoids with Unusual pi-Conjugation Mode
Porphyrin is a typical 18-pi conjugated macrocycle with aromaticity. One of the strategies to dramatically alter the reactivity of porphyrin is to perturbate the aromatic character. In theoretical, porphyrin is expected to produce the corresponding 20pi-conjugated compound by injection of 2 protons and 2 electrons, although the isolation of the 20pi-conjugated from is difficult because aromaticity is lost. We successfully isolated and characterized the 20pi-conjugated porphycene, a structural isomer of porphyrin, by introducing CF3 groups in the periphery of the porphycene framework. On the basis of kinetics and thermochemical analysis, we proposed that the production of the antiaromatic compound proceeds via a concerted proton-electron transfer (CPET) mechanism.
【In detail】Org. Lett. 2007, 9, 5303-5306.; J. Org. Chem. in press.
8. Porphyrinoid Chemistry in Protein Matrices
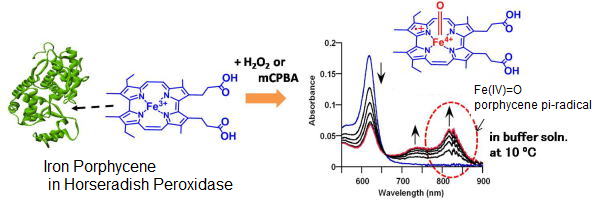
Metal complexes included in protein matrices show us unique reactivities because of the special reaction environments and steric protection by protein matrices, which are difficult to see for the corresponding model compounds in organic solvents. For example, horseradish peroxidase (HRP), a heme-containing oxidase, has the remarkable catalytic oxidation activity, where the observable high-valent species(Fe(IV)=O) are generated. We successfully detected the Fe(IV)=O species of an iron porphycene by incorporating it into HRP and characterized by UV-vis spectroscopy. The reactivities of these high-valent species were also investigated by kinetic measurements.
【In detail】J. Am. Chem. Soc. 2007, 129, 12906-12907; Chem. Asian J. 2011, 6, 2491-2499
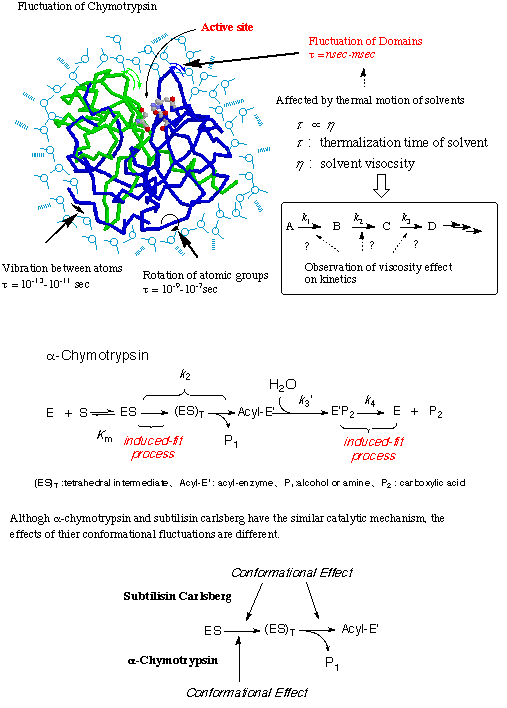
9. Study on Reaction Mechanism of Serine Proteases from the Viewpoint of Enzyme Fluctuation
It has been proposed that extraordinary acceleration of a chemical reaction by an enzyme is achieved not only by chemical catalytic mechanism at the active site but also by such physical actions as conformational fluctuations of an enzyme. According to induced-fit theory, multi-point interactions between an enzyme and a suitable substrate induce the conformational change of an enzyme during thermal fluctuation so that reactive groups at the active site are forced to take the optimal position for the catalysis. The fluctuations of an enzyme is regarded as Brownian motions and affected by medium viscosity. Then, we investigated effects of medium viscosity on kinetic parameters of serine proteases (e.g. alpha-chymotrypsin etc.) and elucidated the significance of conformational fluctuation of the enzyme for their catalytic activity. Based upon the experimental data, we proposed the novel reaction mechanism of serine proteases from the viewpoint of enzyme fluctuations. In a fluctuation-controlled reaction, the thermal equilibrium between the ground state and the transition state, the assumption in Eyring's transition state theory (TST), fails. Therefore, the experimental data in this research were applied to Sumi-Marcus theory in which the two-dimensional potential is proposed.
【in detail】 J. Chem. Soc. PerkinTrans 2 887-891 (2000) ; Bull. Chem. Soc. Jpn 71, 2187-2196 (1998)