Research
We are interested in the development of functional organic materials, such as organic semiconducting materials for photovoltaic cells and organic thin-film transistors (OTFT), highly fluorescent materials, etc. on the basis of organic synthesis. In particular, acenes and porphyrinoids are our current target compounds.
1. Photochemical synthesis of acene derivatives
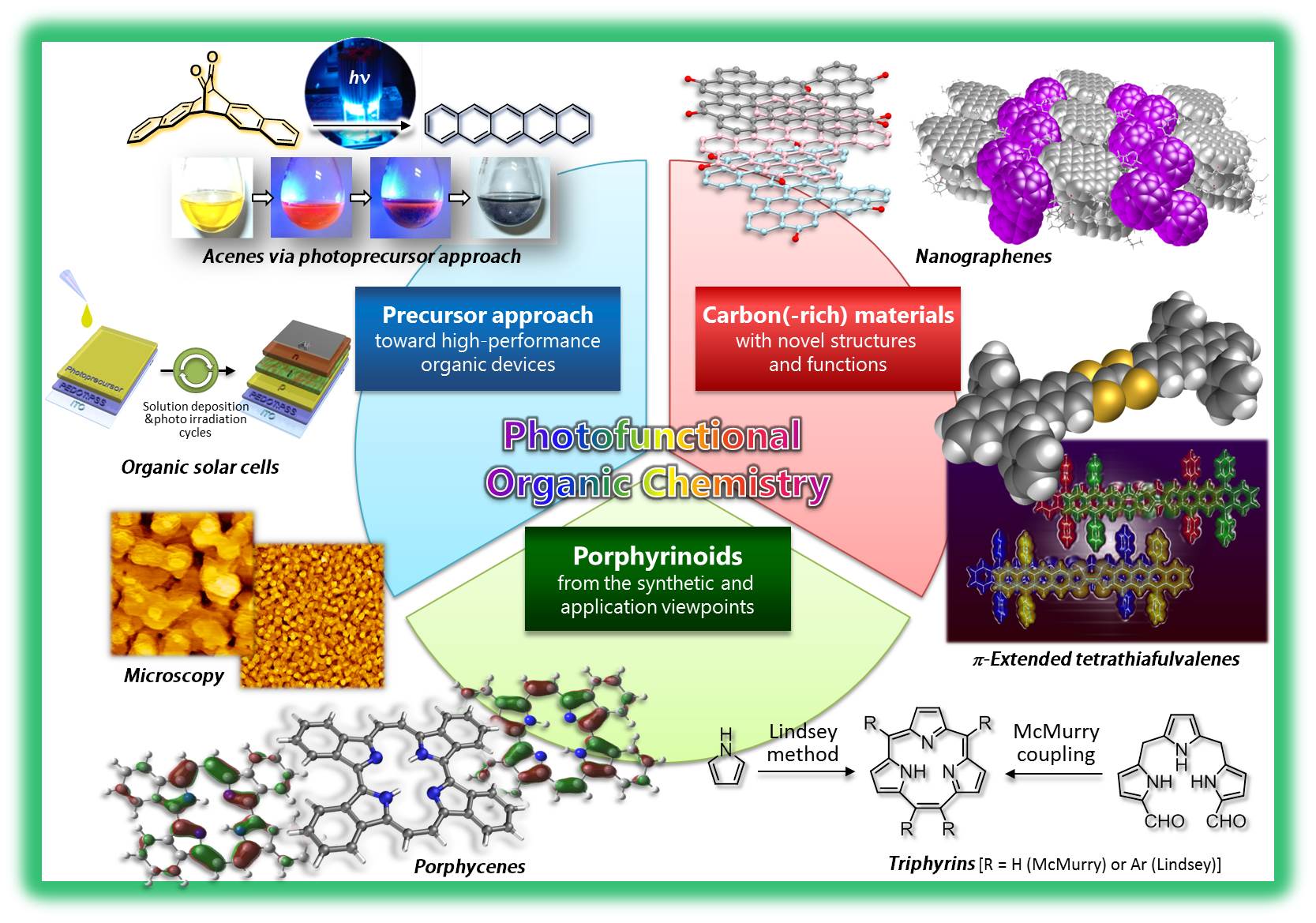
Acenes are defined as polycyclic aromatic hydrocarbons (PAH) consisting of linearly fused benzene rings. We have succeeded to prepare pentacene from a soluble diketone precursor (PDK) by photoirradiation in solution and in film (see video below). This was the first pentacene precursor which can be converted to pentacene by photoirradiation.[1] We were also successful to fabricate the pentacene-based OTFT by solution process of PDK followed by photoirradiation. This device showed the hole mobility of 0.86 cm2 V–1 s–1. This method can be applied to anthracene-based semiconducting materials which are stable under ambient atmosphere.[2] This procedure, which we call "photoprecursor approach", is useful synthetic method for the preparation of unstable larger acenes and pentacene derivatives. We have reported the synthesis of 1,4,8,11-tetraarylpentacenes from α-diketone precursors by this method.[3]
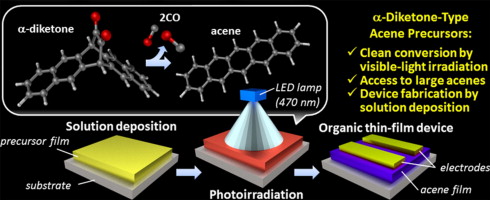
The photoprecursor approach also enables layer-by-layer deposition of different materials by solution-based techniques, given the solubility of photoreaction product is sufficiently low. In addition, it allows for the control of film crystallinity or morphology through modulation of photoreaction conditions.[4] We have been applying this approach to the preparation of p–i–n-type active layers of organic photovoltaic cells (OPVs).[5]
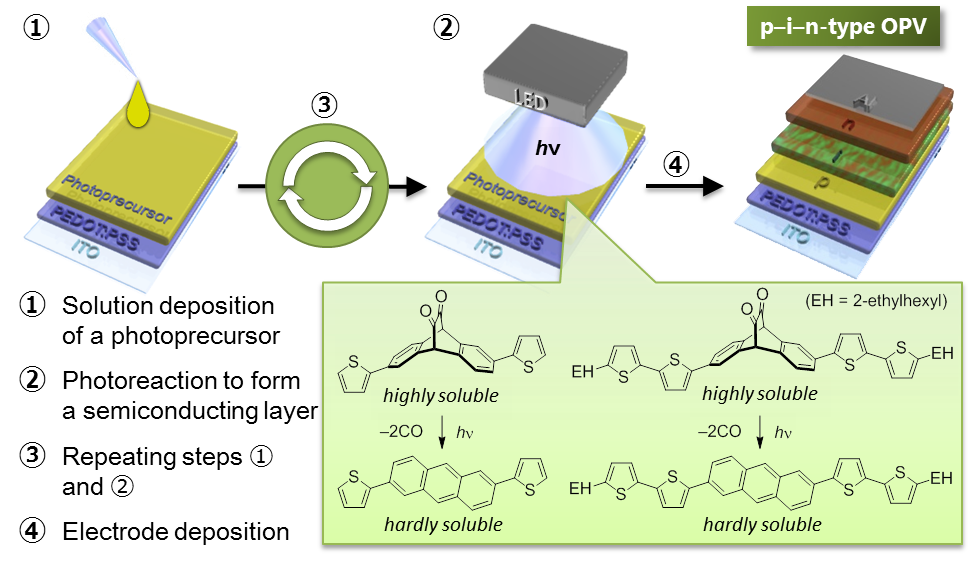
α-Diketone moiety attached to highly fluorescent materials often makes them non-emissive. The diketone moiety can be removed by photoirradiation, then the fluorescence comes again. On the other hand, BCOD (bicyclo[2.2.2]octadiene) unit is converted to benzene by heating. Thus, BCOD-DK can show four different optical performances simply by irradiation and heating.[4]
[1] a) Tetrahedron Lett. 2005, 46, 1981–1983; b) Chem. Eur. J. 2005, 11, 6212–6220; [2] Chem. Commun. 2012, 48, 11136–11138; [3] Tetrahedron Lett. 2010, 51, 1397–1400; [4] Tetrahedron Lett. 2013, 54, 1790–1793.
2. Creation of unique carbon frameworks with remarkable optical/electronic properties
Highly electronically interactive π-conjugated systems have received much attention in light of their potential applications in (opto)electronic devices, sensors, photovoltaic devices, non-linear optical (NLO) materials, and light-harvesting antenna models. On the basis of transition-metal catalyzed coupling reaction, we have prepared an all-56-carbon conjugative tetrabenzoperipentacene.[1] This structure was confirmed by single crystal X-ray diffraction analysis and encouraged possible applications for nano-graphene models. From the X-ray structure, it is revealed that three molecules make a triple-layered cluster via π-stacking manner in which each layer rotates by 120°, considered as a petit beta-graphite. The Stokes shift is extremely small value as about 10 cm–1, indicating its remarkably rigid framework. The tetrabenzoperipentacene has exhibited reversible five-electron oxidation waves in cyclic voltammetry, thus revealed the superior redox behavior of the multi-charge storage molecular graphene.
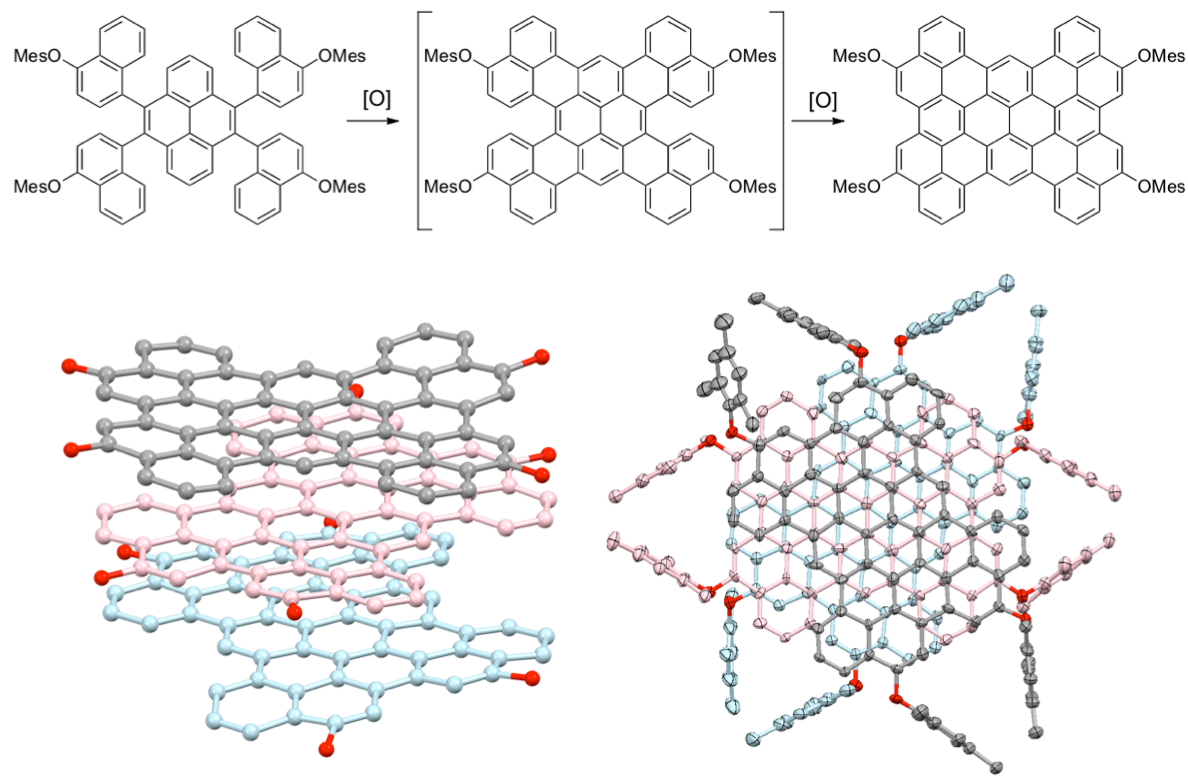
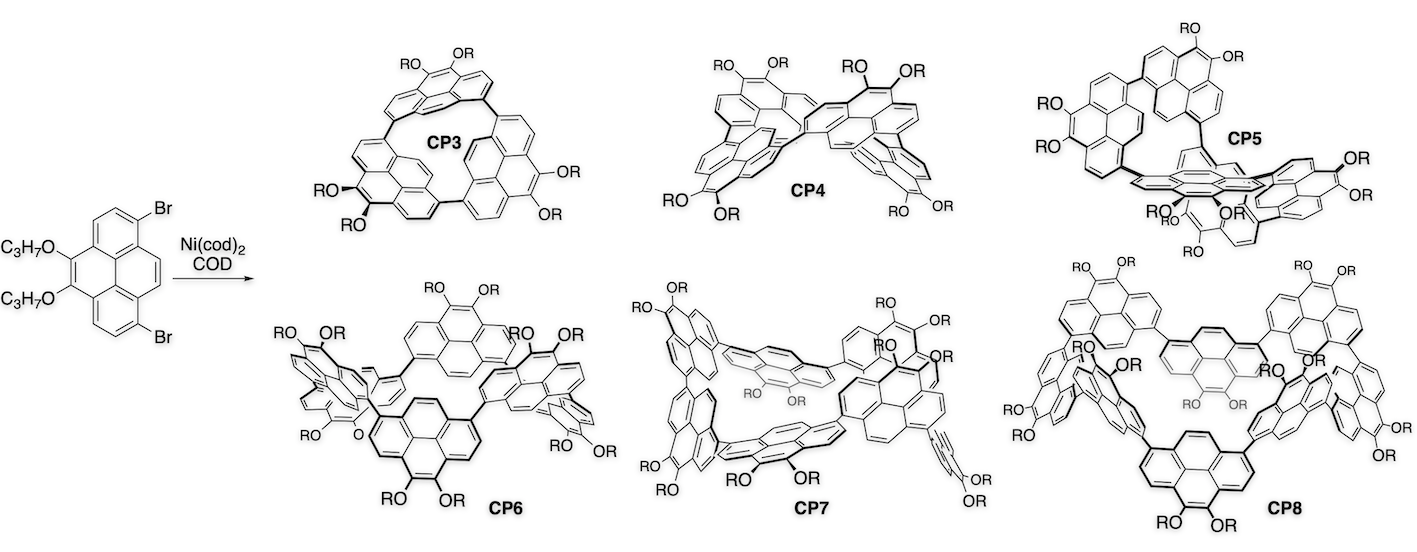
Arylene macrocycles constitute one of the most important shape-persistent oligomers. Owing to their remarkable emissive behavior, we made 1,8-pyrenylene macrocycles and found their structure-dependent unique properties.[2] Coupling of 1,8-dibromopyrene with Ni(cod)2 provided a series of directly-linked cyclic pyrene CP3, CP4, CP5, and higher cyclic oligomers. In case of CP4, four pyrene planes make two π-surrounded large spaces for guest binding. In fact, formation of the 1:1 complex of CP4 with C60 results in one-dimensional chain growing, which was confirmed by X-ray analysis.[3] On the other hand, CP5 represents a new class of compound having an isolable chiral structure, which is achieved without any special intramolecular bonding. Furthermore, asymmetrically pure CP5 shows circular dichroism (CD) and circularly polarized luminescence (CPL) signals.[2] These features are particularly advantageous for future applications in optical devices.
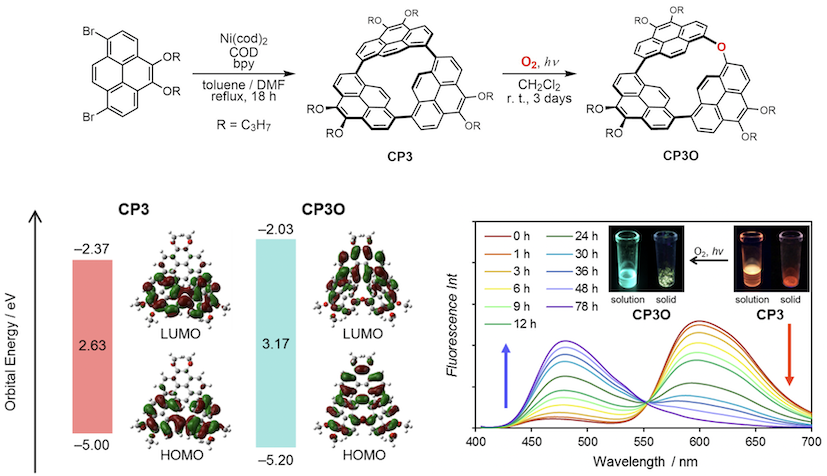
All the cyclic oligomers isolated show intense emission. In particular, CP3 exhibits red-shifted orange emission because of the strain of two pyrenes. A molecule experiences strain when the molecule is held in an energetically unfavorable conformation by intramolecular covalent bonds in comparison to a strain-free reference compound. After cutting off the particular bonds, the strain energy would be released. In this work, we report synthesis of a highly strained cyclic pyrene trimer and its oxygen insertion reaction with a dramatic change in optical properties.[4]
[1] Angew. Chem. Int. Ed. 2015, 54, 8175; [2] Chem. Commun. 2019, 55, 9618; (Selected as an Inside Back Cover) [3] Chem. Lett. 2020, 49, 892; (Editor's chioce) [4] Chem. Sci. 2019, 10, 6785; (Selected as an Inside Back Cover)
3. Synthesis of Acene-based Functional Compounds and Challenge for Bottom-up Synthesis of Nanocarbon Materials
Polyacenes
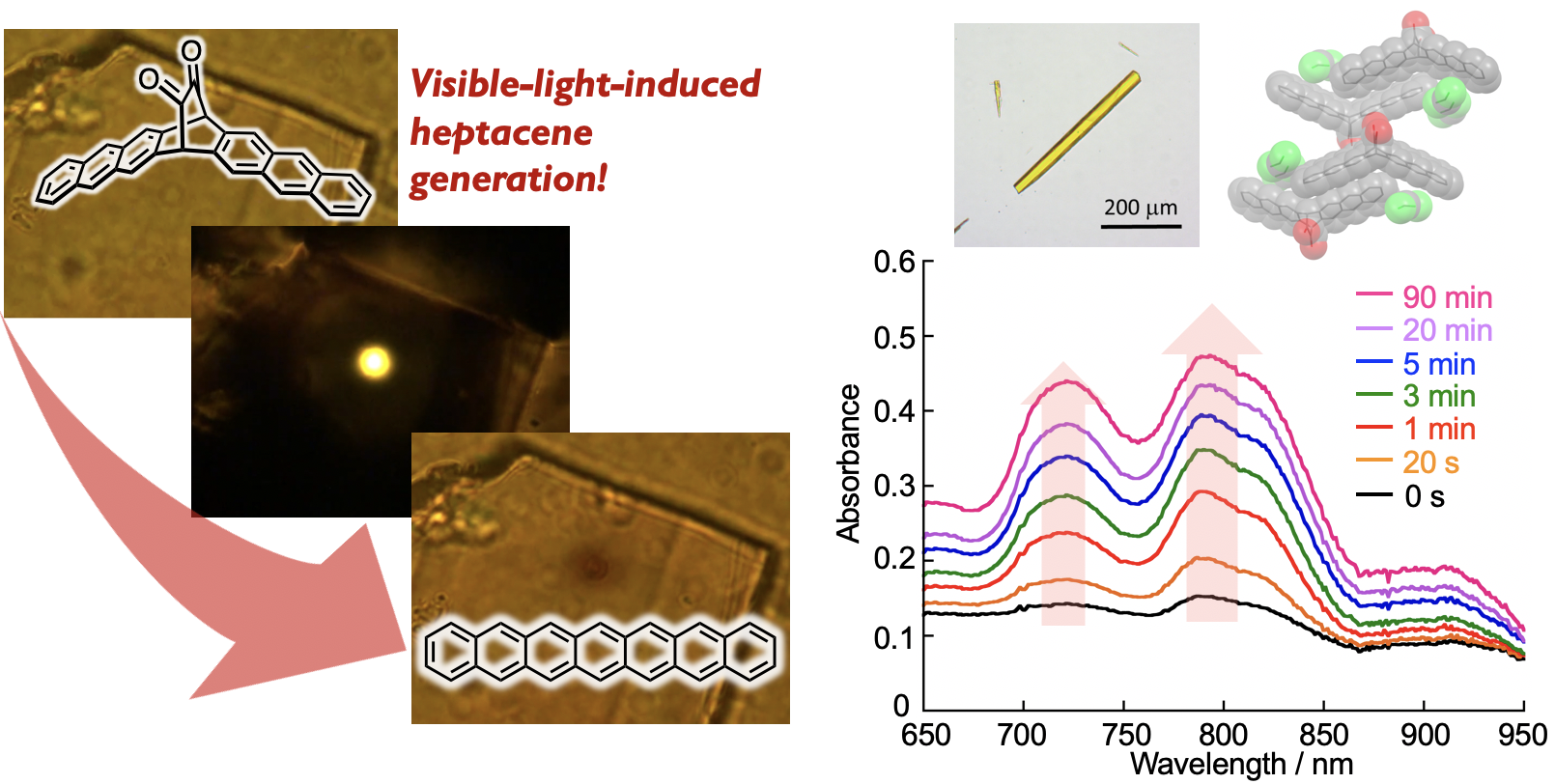
Polyacenes, composed of multiple linearly fused benzene rings, have drawn considerable attention because of their potential utility as organic semiconductor materials. Their intrinsic optical and electronic properties, stemming from their zigzag-edge, render them suitable candidates for exploring magnetism in graphene nanostructures. In addition, they can be precursor molecules for graphene nanoribbons (GNRs) synthesized by bottom-up method. At the same time, however, the zigzag-edged structure of polyacenes results in ultra-narrow HOMO–LUMO gaps with increasing acene length, which causes a decrease in stability. Furthermore, polyacenes are poorly soluble, due to their uncomplicated structure, which lacks functional groups. Currently, we are tackling these synthetic problems by using photochemical precursor method. We reported the synthesis of alpha –diketone–type dibromoheptacene precursors and the on-surface formation of heptacene organometallic complexes on Au(111) under UHV conditions. STM and nc-AFM revealed the formation of Au-heptacene complexes via a selective two-step activation of alpha -diketone-type dibromoheptacene precursors.[1] In addition, we demonstrate the on-surface generation of heptacene and nonacene via visible-light-induced photodecarbonylation of precursors on Au(111) under UHV conditions.[2] STM and nc-AFM unambiguously confirmed their chemical structures. STS measurements together with theoretical calculations indicate that large acenes on an Au(111) surface acquire an open-shell character. (Collaboration with Prof. Roman Fasel@Empa)
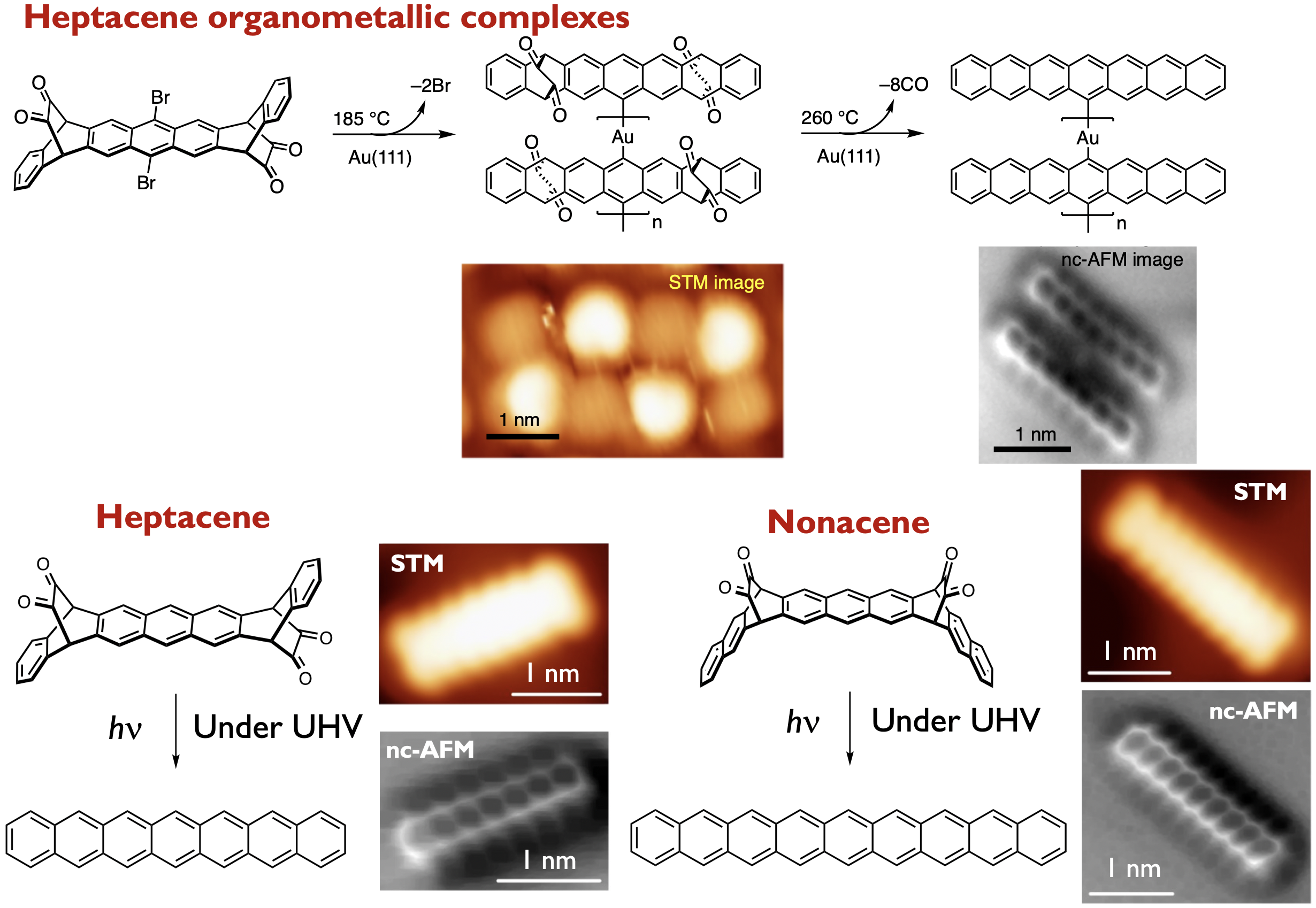
Here in above, such conversions necessitate anaerobic conditions. Consequently, the generation of polyacenes via visible-light irradiation and ambient conditions is considerably challenging, while its success would provide a facile access to the fundamental properties of polyacenes. The isolation of generated polyacenes from the external environment prevents their oxidation. In this context, we have successfully demonstrated the photoconversion of a crystalline phase alpha-diketone-type heptacene precursor into heptacene, with the use of a 488 nm CW laser.[3] Pivotal to the success of this method was the selective irradiation of the crystal interior by using CW laser, with the generated heptacenes being shielded by heptacene precursors on the outer regions of the crystal. This strategy provides efficient access to otherwise inaccessible compounds. We believe that this approach is of general relevance for the synthesis and characterization of unstable molecules. (Collaboration with Prof. Masuo@Kwansei-Gakuin University)
Graphene Nanoribbons (GNRs)
Small acene derivatives can be precursor molecules for GNRs. Specifically, we are interested in edge-functionalized GNRs which have been predicted to show attractive electronic properties that have yet to be demonstrated experimentally. With these in mind, we are working towards synthesis of edge-fluorinated GNRs and the reaction mechanism analysis.[4] During this project, interpolymer self-assembly of bottom-up GNRs has been realizied by using fluorinated acene precursors, improving on-current of GNR-based FETs.[5]
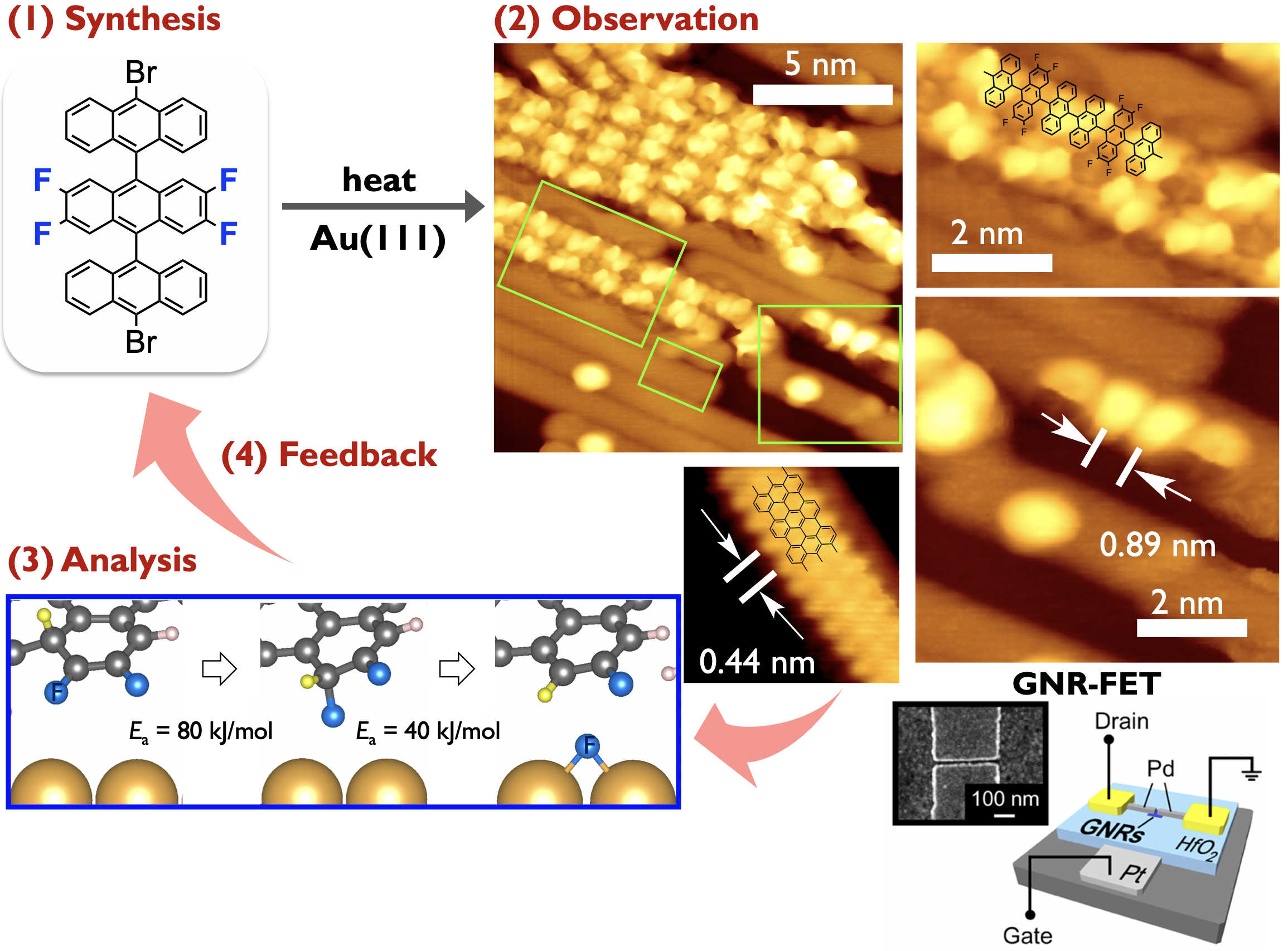
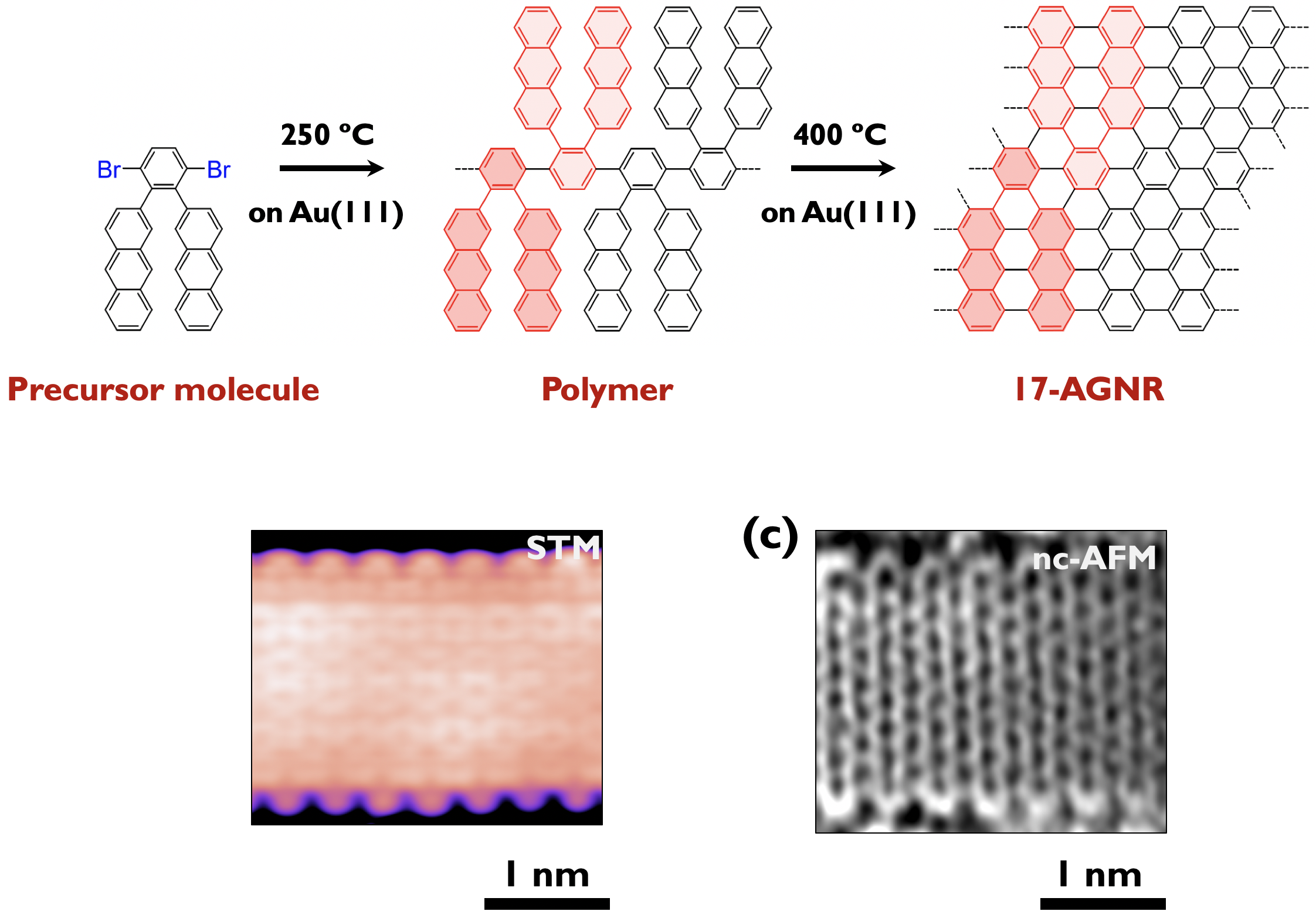
It is reported that armchair-edged GNRs (AGNRs) exhibit width-dependent bandgaps. They can be classified into three subfamilies (3p, 3p + 1, 3p + 2), their band gaps being inversely proportional to the width of those families. Basically, wider armchair-edge graphene nanoribbons belonging to the 3p + 2 subfamily have the smallest bandgaps among different graphene nanoribbons, having considerable potential to be exploited in GNR-based devices. Nevertheless, the bandgaps of AGNRs synthesized experimentally are relatively large (> 1 eV) so far. Recently, we have successfully prepared 17-atom-wide AGNRs (17-AGNRs) by on-surface-assisted polymerization and subsequent cyclodehydrogenation of precursor molecules on Au(111).[6] We reveal that 17-AGNRs have the smallest bandgap, 0.2 eV on Au(111). The successful synthesis of 17-AGNRs is a significant step toward the development of GNR-based electronic devices (Press release [7]). (Collaboration with Fujitsu lab.)
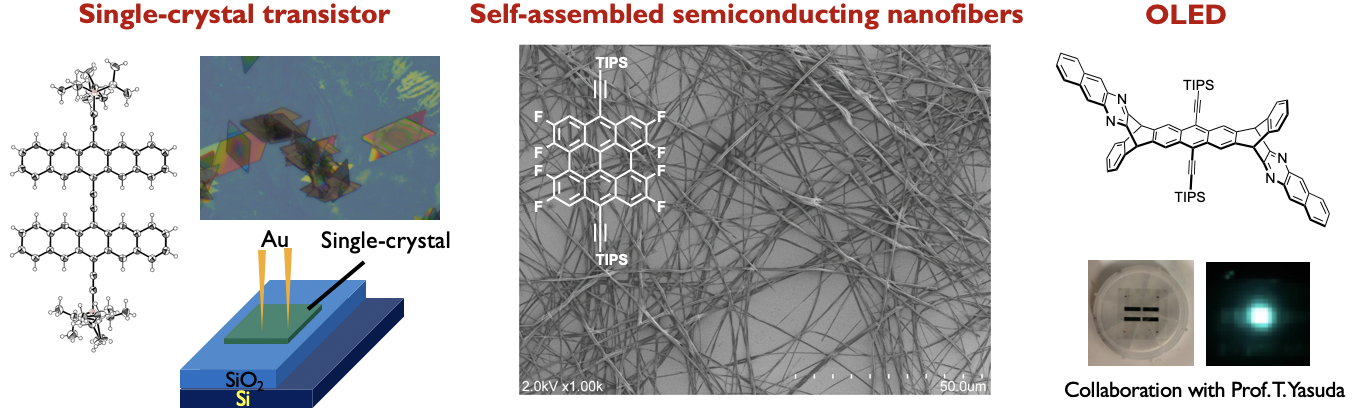
Acene-based Functional Materials
Our research also focuses on molecular and supramolecular systems with charge transport property and unique fluorescence by using acene-based functional materials. Current functions of interest are FET and/or single crystal FET devices, and OLED with pi-extended acene derivative.[8–10] Specifically, a rigid and planar ethynylene-bridged pentacene dimer was synthesized from pentacenequinone via two steps, skipping the conventional stepwise approach (it needs more than 7 steps!). Highly crystalline dip-coated films exhibited average hole mobility of 0.24 cm2 V–1 s–1, comparable to that of the single-crystal organic field-effect transistors. This discovery and understanding of the reaction for the facile synthesis of ethynylene-bridged pi-conjugated systems enables to the synthesis of a wide range of organic semiconducting materials. Development of unique ethynylene-bridged pi-conjugated systems by using this reaction mechanism is ongoing in our group.
[1] J. Am. Chem. Soc.. 2017, 139, 11658–11661.; [2] Nat. Commun.. 2019, 10, 861.; [3] Chem.–Eur. J.. 2020, 26, 15079–15083. (Selected as a HOT Paper); [4]ACS Nano. 2017, 11, 6204–6210.; [5] ACS Appl. Mater. Interfaces, 2018, 10, 31623–31630.; [6]Commun. Mater. , 2020, 1, 36. [7] https://www.sciencedaily.com/releases/2020/06/200630111440.htm; [8]Chem.–Eur. J., 2018, 9, 14916–14920. [9]Chem.–Eur. J., 2017, 23, 7000–7008 (Selected as a HOT Paper and a Frontispiece). [10]Chem.–Eur. J., 2019, 25, 15565–15571.
4. Novel porphyrinoids
Porphycene
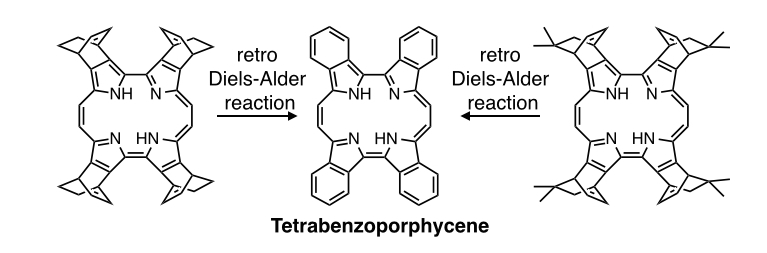
Porphycenes, constitutional isomers of porphyrins, have lower symmetry than porphyrins and display intense absorption bands in the NIR regions due to the decreased LUMO level compared to porphyrin. In spite of the interesting electronic properties, the study of porphycenes has not been as popular as porphyrins. We have successfully synthesized benzo- and naphthoporphycenes by a retro Diels-Alder method from the corresponding bicyclo[2.2.2]octadine (BCOD) precursors and demonstrated the control of the fluorescence properties and the aromaticity by the substituents of the porphycene core[7]. In addition, free-base tetrabenzoporphycene showed herringbone structure in the crytal state, while its zinc complex made a hexahedral box-structure with six coordinated pyridine molecules inside. Dodeca-substituted porphycenes were also prepared for the first time[8]. These porphycenes can be applied to organic devices such as OTFTs and OSCs.
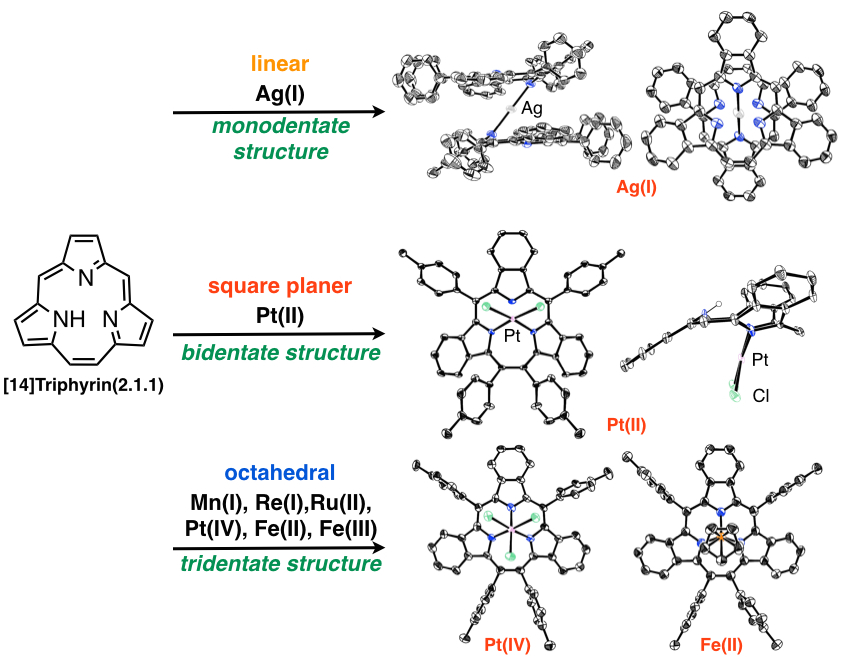
[14]Triphyrin(2.1.1)
Triphyrins, ring-contracted porphyrin analogues, consist of only three pyrrole and methine carbon atoms. Triphyrins are new family of porphyrin analogues. We have succeeded in preparation of the [14]triphyrins(2.1.1) by a modified Lindsey method[9] and an intramolecure McMurry coupling method[10]. The triphyrin has nearly planar structure with 14π aromatic system and was obtained as freebase form, while the previously reported triphyrins have mainly been coordinated with boron(III) atom and had bowl-shaped structure. [14]Triphyrins(2.1.1) can react with various metal ions to afford the manganese(I), rhenium(I), ruthenium(II), platinum (II) and platinum (IV) complexes. With coordination of the metal ions, the structures were changed from nearly planar to bowl-shaped structure and metal ions were placed on the top of macrocycles due to the small cavity. We have also synthesized thiatriphyrin (TTP), which includes one thiophene ring in place of one of three pyrrole rings. The neutral TTP was too unstable to be isolated and was highly reactive with alcohol to afford alkoxy-attached thiatriphyrin. Interestingly, it was converted to protonated TTP with 14π aromaticity by addition of acid to remove alcohol[11]. We have developed the synthetic methods, metal complexes and core-modification of the triphyrins to develpo the new structure and functionality.
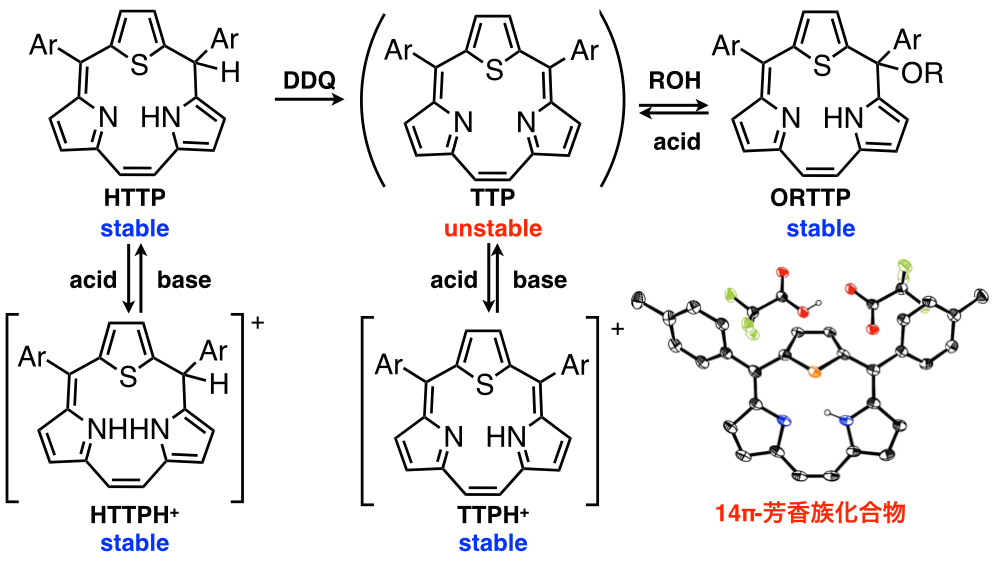
[7] Chem. Eur. J. 2009, 15, 10060–10069; [8] Chem. Eur. J. 2011, 17, 3376–3383; [9] a)JACS. 2008, 130, 16478–16479; b)Chem. Eur. J. 2011, 17, 4396–4407; [10] Chem. Commun., 2011, 47, 722–724; [11] Angew. Chem. Int. Ed. , 2013, 52, 3360.






